All polyol classes used in polyurethanes have a set of unique attributes that make them useful as the soft-segment in specific polyurethane elastomer applications.
For example, polytetramethylene ether glygol (PTMEG) polyols exhibit excellent hydrolytic stability, best in class resiliency/rebound, impingement abrasion resistance, good dynamic properties, and low temperature flexibility. The aliphatic polyester polyols, on the other hand, exhibit excellent tensile properties, chemical resistance, cut resistance, tear strength, and high temperature stability. Compared to the standard polyester polyols, polycaprolactone polyols provide enhanced hydrolytic stability and chemical resistance, a wider range of thermal (low & high temperature) properties, impact resistance, cut, tear and chip resistance, sliding abrasion resistance, and excellent durability.
The polycarbonate diols, the subject of this article, represent the next generation of ultimate performance diols for polyurethane elastomers. Their combination of high resistance to heat, hydrolysis, weather, and abrasion, aging stability, consistent performance and extreme durability is unattainable by other polyols.
Attributes of Polycarbonate Diols in Polyurethanes
Polycarbonate diols feature several valuable attributes for use in the development of polyurethanes:
- Excellent hydrolytic stability (except in base) & low water absorption
- Maximum temperature stability; low temperature flexibility
- High chemical & oil resistance
- Weatherability & UV resistance
- Superior environmental stress crack resistance
- Low yellowing and high gloss retention
- Superior durability, impact and abrasion resistance
- High mechanical properties
- Long-term retention of designed properties in aggressive environments
Performance Often Overrides Cost Concerns
Cost is a major consideration in the selection of the soft-block polyol in tailoring the property profiles of polyurethanes. While polycarbonate diols are higher in cost than the other classes of polyols, they are increasingly used in polyurethane coatings, adhesives, and cast elastomer applications requiring extreme durability, due to their performance-based properties
Specific application arenas include waterborne polyurethane dispersions, oil field and mining polyurethane cast parts, pipe linings and exterior coatings, electrical/electronic encapsulation, artificial leather coatings, optical devices, medical devices, elastomeric rollers and thermoplastic polyurethanes (TPUs).
The global market for the aliphatic polycarbonate diols is estimated at nearly 110 million pounds (50,000 MT) with a current growth rate above five percent, which is higher than the CAGR growth rate estimate for the polyurethane industry as a whole. The largest consuming region is Asia (45%), followed by North America (25%) and Europe (15%). From a market perspective, polycarbonate diols can be subdivided into crystalline-solid type polyols and liquid type co-polycarbonate diols, with the solid type diols holding the higher market share at about 65 percent.
The Chemistry of Polycarbonate Diols
Structurally, polycarbonate and co-polycarbonate diols (liquid type) are linear, aliphatic polyols with the carbonate linkages. The structure below shows the molecular design of the 1,6-hexanediol polycarbonate diol. The carbonate linkage in a polymer chain exhibits good stability.

Chemists produce aliphatic polycarbonate diols in a two-stage process by the catalyzed (e.g. tetrabutoxy titanium, dibutyltin oxide or bases) reaction of a diol with either dimethyl carbonate (DMC) or diphenyl carbonate (DPC). In the final reaction stage, they remove excess DMC or DPC and mono-alcohol (methanol or phenol) under reduced pressure to uncap the terminal hydroxyl (-OH) functionality. The polycarbonate polyols are primary diols and exhibit good isocyanate reactivities.
The polycarbonate diols exhibit the highest hydrolytic stability of the polyester polyols because they absorb only low levels of moisture and they generate CO2 on hydrolysis without producing an acidic moiety. Acid moieties autocatalyze further hydrolysis in the soft-block of the polyurethane.
Properties of Cast Polycarbonate Diol Polyurethanes
As indicated above, the polycarbonate diols (PCDs) are the premier polyols used in polyurethanes, albeit at a higher cost. We prefer to compare polycarbonate diol to our polycaprolactone polyols in PURs because this comparison best exemplifies the high performance of polyurethane elastomers based on these two polyol classes.
In the table below, we compare a 2000 MW HDO polycarbonate diol Placcel® CD 220 from Daicel Corporation with Placcel® 220N, a 2000 MW EG initiated narrow MW Distribution polycaprolactone diol, in a cast MDI polyurethane. The PCD-based elastomer (2) had higher hardness and a much higher Tg when compared to the Placcel® 220N based elastomer (1) with the same compositional hard segment content of 35.2%. We achieved equivalent hardness when the hard segment content of the polycaprolactone elastomer is raised to 41.6 % (3).
Under the same hard segment content, the polycarbonate diol-based elastomer (2) and the polycaprolactone polyol (1) show similar compression set values, but the PCD 220 elastomer showed a lower abrasion loss. Elastic recovery of the PCL based CPUs were better when measured by stretching samples to 300 % elongation at a deformation rate of 500 mm/min. followed by recovery measurements after 10 minutes from the release of the stress. Some of the data for polycarbonate-urethane likely reflects crystallization in the soft block HDO-PC segment.
Comparative Properties of 2000 MW Carbonate Diols vs. Polycaprolactone Diol based CPUs
| Sample | 1 | 2 | 3 | Method |
| PCL-MDI-BDO 1:3.5:2.4 |
PCD-MDI-BDO 1:3.5:2.4 |
PCL-MDI-BDO 1:4.5:3.3 |
||
| Hard Segment, % | 35.2 | 35.2 | 41.6 | |
| Hardness (A) | 85 | 89 | 89 | ISO 7619-1 |
| Tensile Strength, MPa | 43.2 | 42.2 | 47.0 | ISO 37 |
| Elongation, % | 465 | 329 | 481 | ISO 37 |
| Tear Strength, KN/m | 87.9 | 89.1 | 94.0 | ISO 34 |
| Compression Set (72h/70ºC), % | 26.3 | 26.0 | 18.7 | ISO 815-1 |
| Rebound Resilience, % | 65.4 | 44.8 | 60.8 | ISO 4662 |
| Elastic Recovery, % | 61.7 | 26.7 | 62.6 | * |
| Abrasion, mg | 35.4 | 25.8 | 28.8 | ISO 4649 |
| Glass-Transition, ºC | -27.9 | 7.3 | -23.9 | DSC |
In the figure below, we measured the the storage modulus of the same three samples between -60°C to +190°C. From about 0°C to 150°C the storage modulus of the PCL samples (1) and (3) exhibited better consistency through a wider temperature range than the PCD based elastomer (2), a reflection of the higher Tg of the polycarbonate diol. The high storage modulus of these elastomers is very useful in high load applications.

Dynamic Properties of PCD and PCL based CPUs
The two PCL-based elastomers showed a lower tangent delta (tan δ) in the TMA, which is indicative of the respective Tg’s of the three samples. An elastomer with lower hysteresis exhibits less heat build-up at high speeds, lower rolling resistance, and wet skid resistance. The excellent stress-strain properties and tear properties of both the polycarbonate diol and polycaprolactone diols compares favorably with PTMEG elastomers.
Hydrolytic Stability of Carbonate Polyols vs. other Polyols
Polycarbonate diols exhibit significantly better hydrolytic stability than their adipate polyester polyol counterparts. The figure below compares the tensile strength retention of Placcel® CD 220 polyols, a 2000 MW HDO type polycarbonate diol (*3) TPU, against other polyol families. We measured tensile properties on MDI-based TPU pieces soaked in water at 80 °C for three weeks and four weeks. Particularly noteworthy is the high hydrolysis-resistance of Placcel® CD220 (3). Also shown in the figure are two polycaprolactone diols from the Placcel® 220 N series, PTMEG 2000 (*1); and a 2000 MW 1,6-HDO adipate (*2). The tensile strength of the HDO-PC was relatively unchanged versus the comparable data for the other polyol families.
Tensile Strength Retention of PURs based on Various Polyols

TPU Composition: Polyol/MDI/BDO ratio 1.0/3.5/2.5. OH/NCO ratio 0.95
Tensile Strength Retention of MDI Elastomers
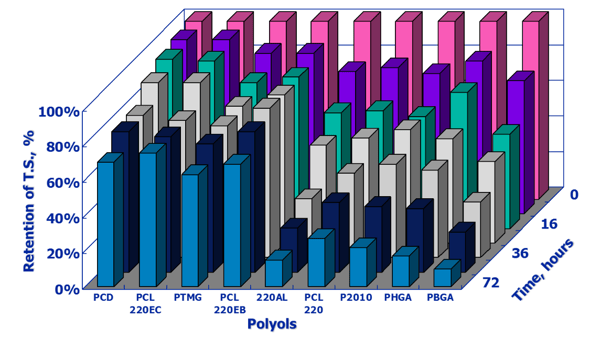
We evaluated tensile strength retention for a wide variety of MDI elastomers based on different classes of polyols under conditions of 105°C and 100 percent relative humidity. We determined tensile strength prior to subjecting the test specimens to the harsh test conditions and then after eight-hour intervals up to 72 hours. The Y-Axis is the tensile strength in MPas.
The molecular design of the polyurethanes was as follows:
- Polyol/MDI/Butanediol in the ratio of 1.0/3.0/2.0
- The OH/NCO ratio 0.95
Under the test conditions, the Placcel CD 220 polycarbonate diol (PCD) and copolymer HDO-PC/polycarbonate diol (PCL220EC) exhibited the highest tensile strength retention and absolute tensile strength under the high temperature and humidity conditions of the test. A 2000 MW HDO adipate (PHGA) and a 2000 MW butanediol adipate (PBGA) exhibited the lowest level of property retention. As would be expected, the polyurethane based on a 2000 MW PTMEG diol also showed good retention of tensile properties, but not as good as the polycarbonate diol elastomers.

The percent retentions of tensile strength are given below in the same chart-format, where the Y-coordinate is now the percent of tensile strength retention. This chart highlights the good property retention of the polycarbonate-based polyurethanes vs. the tensile strength fall-off of the two adipate polyol systems on the far-right side of the chart, The polyol types in the center of the chart are polycaprolactones. As can be observed, the resistance of the polycarbonate-urethane elastomers are orders of magnitude better than adipic acid-based polyester-urethanes.
The Daicel product line of polycarbonate diols is shown below. Placcel® CD210 and CD220 are polycarbonate diols based on 1,6-hexanediol. They are high in crystallinity, exhibit good soft-block chain packing, and afford polyurethane elastomers with high modulus values. As a consequence of their crystallization tendency, the corresponding hexanediol-based polycarbonate-urethanes tend to cold-harden at low temperatures with an increase in Durometer values.
Crystallization also causes the associated polycarbonate-urethanes to lose flexibility at low temperatures. HDO-PCs are available where a third monomer is introduced to reduce the crystallization tendencies of the polycarbonate-urethane elastomers. Placcel®CD205Pl, CD220Pl and 220EC are liquid-type, co-polycarbonate diols that produce polyurethanes with low crystallization tendencies, but also lower modulus values. They are hexanediol-polycarbonate diols with a second diol comonomer, or in the case of 220 ED, caprolactone as the third monomer. These polycarbonate-urethanes show the same favorable balance of resistance to heat, hydrolysis and oxidation, etc.
Placcel® Polycarbonate Diols
| Product | MW | Appearance | OH Value, mg KOH/g |
Acid Value, mg KOH/g |
Water (Wt. %) |
Melting Point, (ºC) | Viscosity, (mPa·s, at ºC) |
| CD210 | 1000 | Wax | 115.5 | 0.02 | 0.004 | 40-48 | 415, at 75 |
| CD220 | 2000 | Wax | 55.7 | 0.03 | 0.004 | 47-53 | 2590, at 75 |
| CD205PL | 500 | Liquid | 230.6 | 0.05 | 0.005 | NA | 1400, at 25 |
| CD220PL | 2000 | Liquid | 57.6 | 0.03 | 0.006 | NA | 4615, at 60 |
| 220EC | 2000 | Liquid | 56.5 | 0.02 | 0.004 | NA | 1055, at 75 |
Contact Gantrade to Discuss Your Polyurethane Needs
To understand which polyol is best suited for your unique needs, partner with Gantrade. Our wealth of technical knowledge and expertise, combined with our broad product line, can guide you to tailoring the best polyurethane solution for your applications. Gantrade’s urethane platform of specialty polyols, chain extenders, and curatives provide a broad range of possibilities to achieve your high-performance polyurethane requirements. Contact Gantrade today.